
Thus, in his first paper on wave mechanics, Schrödinger spoke of his theory as representing: “some vibrational process in the atom, which would more nearly approach reality than the electronic orbits, the real existence of which is very much questioned today”.
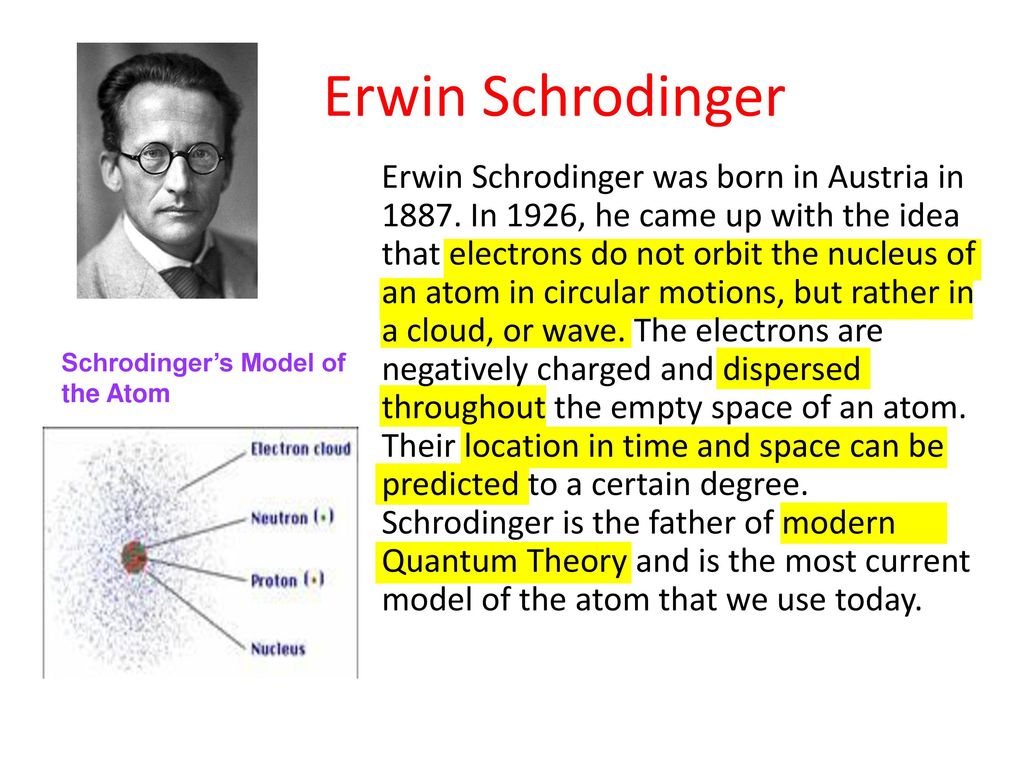
Werner Heisenberg was born in Würzburg, Germany on December 5, 1901. Published the inspiring books 'What Is Life', 'Nature and the Greeks', and 'My View of the World'. The quantum mechanical model has similarities with the Niels Bohr atomic theory in terms. This theory uses mathematical equations to explain the possibility of finding electrons in certain positions. After earning his PhD in physics he was drafted into WWI for the Austria-Hungarian military forces. In 1927, Erwin Schrodinger, an Austrian scientist, put forward an atomic theory called quantum mechanical model of the atom. The great advantage of wave mechanics, Erwin Schrödinger repeatedly emphasized, was that it appeared to promise a visualizable, realistically interpretable description of atomic structure. Erwin Schrodinger was born in Vienna, Austria on August 12, 1887.

Matrix mechanics was a rather abstract and predominantly mathematical theory, which had deliberately refrained from putting forward claims about the unobservable world of atoms. This atomic model is known as the quantum mechanical model of the atom. Schrödinger used mathematical equations to describe the likelihood of finding an electron in a certain position. the Schrdinger wave equation to describe the behavior of electrons in atoms. Description: In 1926 Erwin Schrödinger, an Austrian physicist, took the Bohr atom model one step further. His new theory was presented as an alternative to the theory of matrix mechanics, which had been formulated by Werner Heisenberg along with Max Born and Pascual Jordan some months previously. Schrdinger formulated the theory of wave mechanics, which describes the. In 1926, when he was already a doddering thrty-eight, he was still capable of developing a new quantum theory of atomic structure. Learn vocabulary, terms, and more with flashcards, games, and other study. To explore more similar hd image on PNGitem. SCHRODINGERS ATOMIC THEORY Defined an orbital of an atom as: The region of space that surrounds a nucleus in which two electrons may randomly move.

ERWIN SCHRÖDINGER ATOMIC MODEL DOWNLOAD
The Viennese scientist Erwin Schrödinger became world famous at a relatively late age, that is, for a theoretical physicist active during the 1920’s. Start studying Development of the atomic theory: Erwin Schrodinger (1887-1961). Transparent Atom Electron - Erwin Schrdinger Atomic Model, HD Png Download is free transparent png image. Boston Studies in the Philosophy of Science


 0 kommentar(er)
0 kommentar(er)
